As one of the most common chronic diseases, there are nearly 400 million asthma patients worldwide, and more than 60 million asthma patients in China. In this concern, prevention and treatment of asthma is in the spotlight of public health and medical care issues. More than half of asthma patients are caused by type 2 immune response dominated by allergic-induced TH2 cells. Many immune cell components including eosinophils play important roles in allergic inflammation. The main pathological features are eosinophils infiltration, mucus high secretion and airway hyperresponsiveness are. To find the key mechanism of eosinophils regulating allergic inflammation, research team are led by Prof. SHEN Huahao, Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital of Zhejiang University School of Medicine and Prof. YING Songmin,School of Basic Sciences/the Second Affiliated Hospital. explore about the pathogenesis of asthma entitled Eosinophil-derived chemokine (hCCL15/23, mCCL6) interacts with CCR1 to promote eosinophilic airway inflammation The new finding is published in Signal Transduction and Targeted Therapy.
In this study, they established chemokine CCL6 knockout mice for the first time and explored the functional role of eosinophil-derived mCCL6 in the pathology of allergic inflammation. It is confirmed that CCL6 interacts with G protein-coupled receptor (GPCR) CCR1, constituting a feedforward loop of asthma exacerbation. Targeting CCR1 can inhibit the differentiation of eosinophils and airway inflammation in vivo and in vitro, revealing that targeting the CCL6-CCR1 axis can be a new therapeutic target for clinical treatment of asthma point.
The key regulatory role of CCL6-CCR1 axis in asthma inflammation
Chemokines CCL6/15/23 belong to the NC6 family. Prof. SHEN’s team found that CCL6/15/23 are mainly secreted by eosinophils and confirmed that CCL6 can directly activate CCR1 and its downstream signaling pathways. Lack of CCL6 in mice can significantly alleviate the pathological manifestations of eosinophil infiltration, airway inflammation, and mucus hypersecretion in asthma inflammation, and the differentiation of eosinophils in the bone marrow is also inhibited. The human ortholog of CCL6, CCL15 and 23, were also significantly increased in the plasma of asthma patients, supporting the clinical significance of this finding.
CCL6-CCR1 signal axis as a new target for asthma treatment
In this study, the research team found that CCL6 activates CCR1 coupled Gαi protein and phosphorylation of downstream signaling pathway proteins, thereby promoting HSC activation, providing reliable evidence for the function of CCL6-CCR1 signaling axis in asthma. These findings not only provide new insights into the regulatory role of eosinophils in asthma inflammation, but also provides a therapeutic target of chemokines and receptors in asthma treatment.
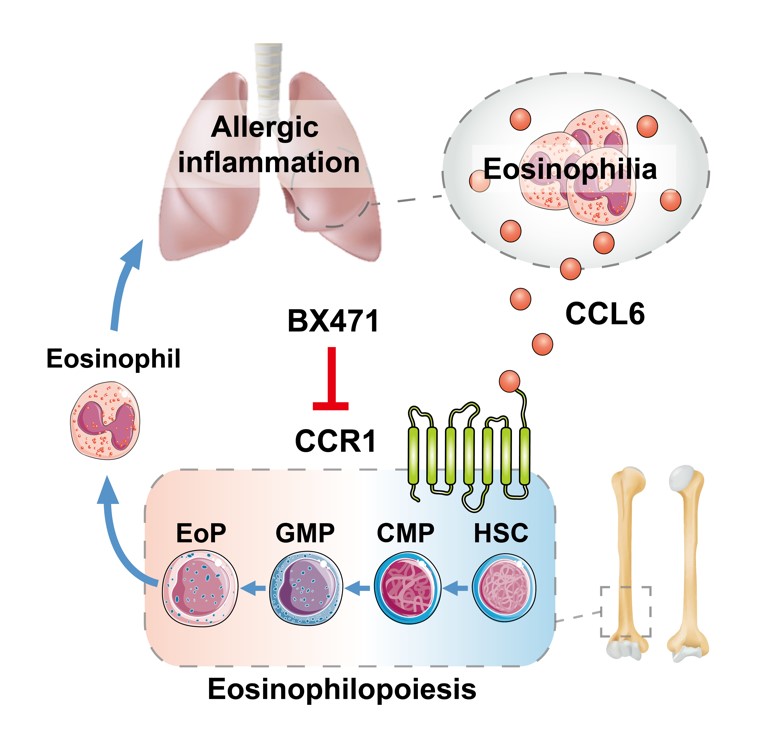
Schematic diagram: CCL6-CCR1 signaling axis regulates asthma inflammation.

Leaders of research team: Prof. YING Songmin (left), Prof. SHEN Huahao (front).